


Organic chemistry
What you need to know
Reflections and Exam tips
Organic chemistry
Organic chemistry is the study of carbon compounds.
Organic chemistry is so complex because...
Carbon atom form very strong covalent bonds
Carbon-carbon bonds can be single, double or triple
Carbon atoms in organic compounds can be arranged in many different forms (isomerism) such as straight chains, branched chains or rings (cyclic or aromatic)
Other elements/groups of elements can be placed on the carbon atoms to form different function groups e.g OH is functional group for alcohols.
Alcohols
Alcohols are not hydrocarbons and contain –OH as functional group.
First few examples are methanol (CH3OH), ethanol (C2H5OH) and propanol (C3H7OH).
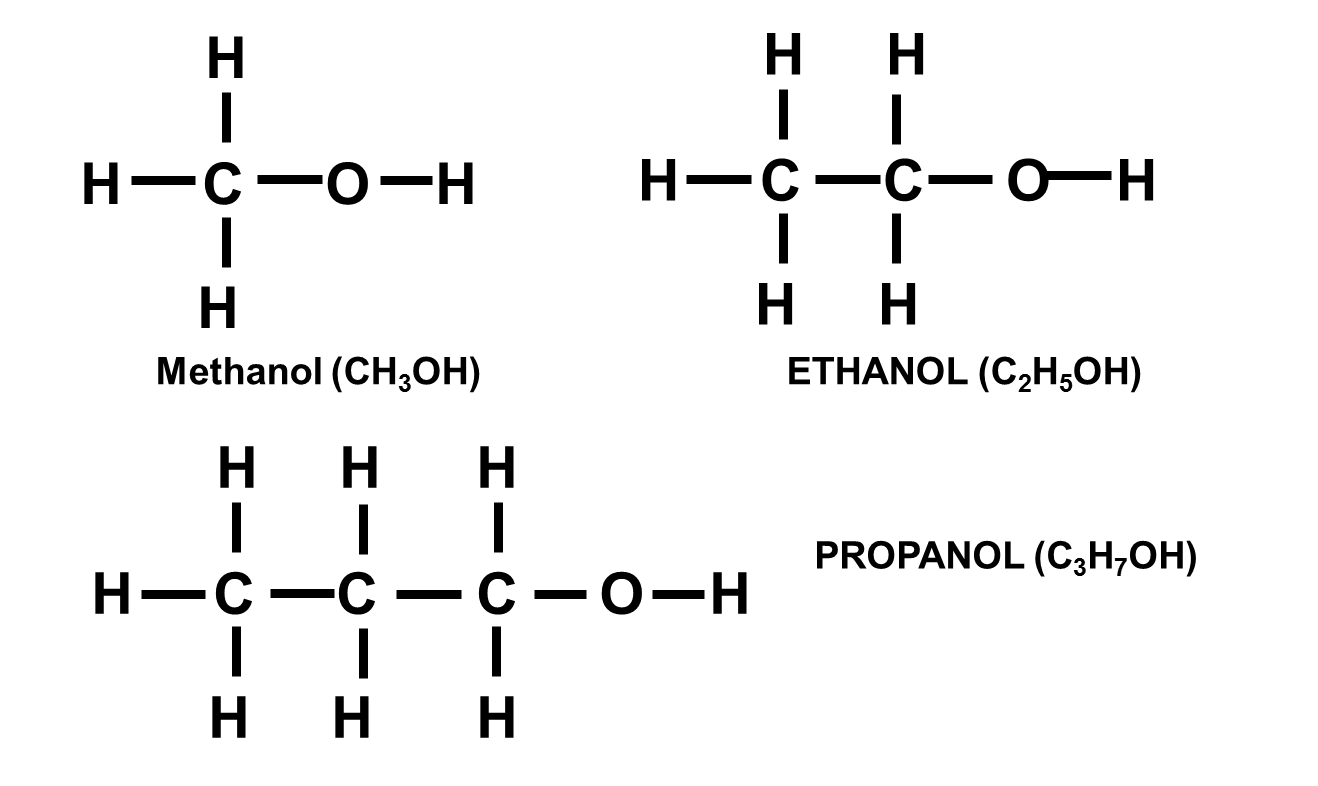
The general formula is … CnH2n+1OH
Combustion
Alcohols make useful fuels just like hydrocarbons.
Ethanol from fermentation is a carbon neutral fuel as the carbon dioxide produced is taken by the next crop of plants.
Ethanol can be made by fermentation of plant materials (renewable).
Ethanol + Oxygen ——> Carbon dioxide + Water
C2H5OH + 3O2 ——> 2CO2 + 3H2O
Esterification
Alcohols (OH) react with carboxylic acids (COOH) to produce ESTERS and water.
Ethanoic acid + Ethanol <--> Ethyl Ethanoate + Water
CH3COOH + C2H5OH <--> CH3COOC2H5 + H2O
The reaction is reversible.
Esters have characteristic smells (used in flavourings).
Reaction with sodium
Alcohols react with sodium and hydrogen gas is produced. Unlike the reaction of sodium with water, the reaction is less vigorous.
Sodium + Ethanol ——> Sodium ethoxide + Hydrogen
2Na + 2C2H5OH ——> 2C2H5ONa + H2
Fermentation
Conditions: Glucose produced from the hydrolysis of starch, yeast and incubated in a warm environment (not higher than 37°C).
Equation C6H12O6 ——> 2 C2H5OH + 2 CO2
Advantages
- Low energy process
- Uses renewable resources
- Uses simple equipment
Disadvantages
- Slow process
- Produces impure ethanol
- Not continuous (batch) process
Hydration of ethene
Conditions: Ethene obtained from cracking crude oil in the presence of catalyst (phosphoric acid) and high temperature and pressure.
Equation C2H4 + H2O ——> 2 C2H5OH
Advantages
- Fast process
- Pure ethanol produced
- Continuous process
Disadvantages
- High energy process
- Expensive plant required
- Uses non-renewable resources to make ethene
Uses of alcohol
- Used in alcoholic drinks
- Used as a solvent such as industrial alcohol / methylated spirits
- Used as an alternative fuel as petrol substitute in countries with limited oil reserves
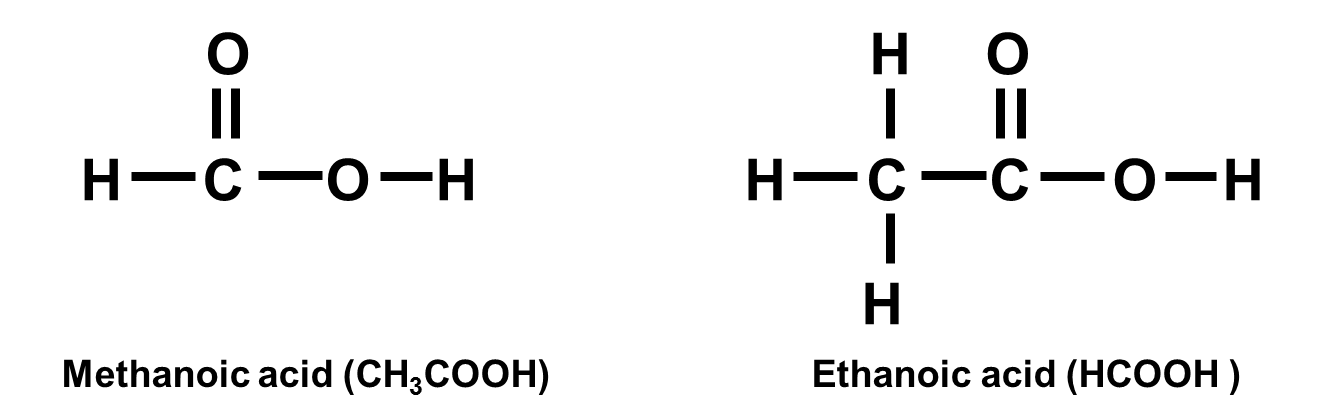
Carboxylic acids
Carboxylic acids contain the functional group -COOH. Examples include methanoic acid (HCOOH), ethanoic acid (CH3COOH) and propanoic acid (C2H5COOH).
Reactions of carboxylic acids
React with alcohols to produce esters in a reversible reaction.
Uses of carboxylic acids
Vinegar contains ethanoic acid which is used in the manufacture of rayon.
Oranges and lemons contain citric acid
Aspirin is a carboxylic acid which is used for pain relief and prevention heart attacks.
Vitamin C contains ascorbic acid which is used for pain relief and prevention heart attacks.
Esters
Esters are formed by replacing the H on the COOH of a carboxylic acid by a carbon atom group to give the functional group COOC.
Examples include methyl methanoate (HCOOCH3) and methyl ethanoate (CH3COOCH3).
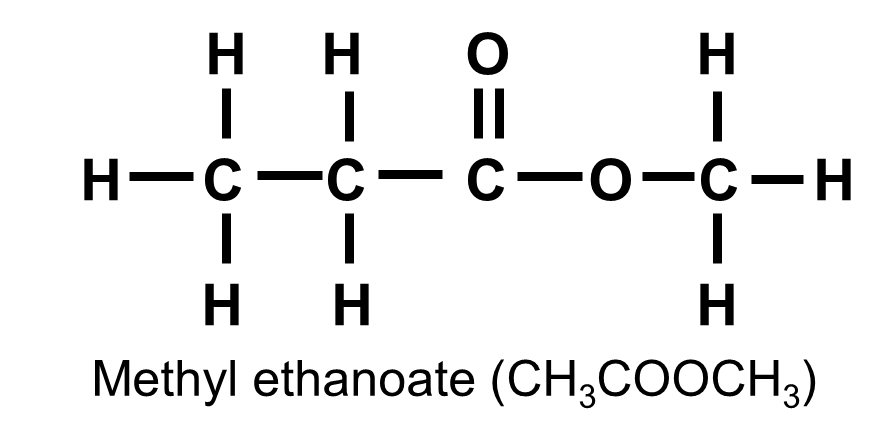
Esters are used as flavourings due to their fruity odours.